Understanding modern brain imaging technologies...
A Primer on Brain Imaging
The understanding that the brain is a vital organ is as widespread as it is old. After all, trepanning, the act of removing parts of the skull to get access to the brain, is the oldest surgical procedure for which we have archaeological evidence. Writings that confirm that people were aware of its specific significance date back 2500 years when Alcmaeon of Croton in the 5th century BC identified the brain as the most important organ for sensation and as the seat of emotion and cognition. However, it took until the dawn of the 20th century until we developed brain imaging techniques to watch it in action.
In this article, I will briefly list and explain the most common modern brain imaging techniques and give an overview over the practical applications and future directions of brain reading.
Indirect Measures
Two of the most popular brain imaging modalities are so-called indirect measures of brain activity. Rather than observing the direct electromagentic discharges of neural firings, they monitor changes in the distribution of oxygen or glucose in the brain which accompany changes in large-scale neural network activity. This approach leverages the observation that, as activity changes, so do the energy-demands of active neurons. Specifically, as neuronal activity in one part of the brain increases, active neurons are in need for oxygen and glucose to replenish expended energy, as they can only store energy for briefest moments of activity.
This replenishment of expended energy is enabled by the brain supplying affected regions with additional glucose and oxygen in its blood supply. However, as an increased total blood supply would cause brain swelling which is constrained by the skull, it keeps the level of blood mostly constant and just reroutes it to active regions on the fly. Specifically, in response to the increased activity, some neurons start producing more nitric oxide which causes blood vessels to dilate in the region. This in turn increases the regional blood supply which carries more oxygen, and glucose to the affected active brain region.
Observing the changes in distributions of oxygen and glucose hence allows for an indirect tracking of changes in brain activity.
One indirect measure of brain activity, called Positron Emission Tomography (PET) works by the injection of a radioactive isotope (of glucose, for example) into the bloodstream. As the isotope will emit high-energy photons, sensitive photon-sensors around the head of the human inside the PET scanner will then allow to calculate the precise isotope position and hence give an indirect account of brain activity changes as blood flow changes reroute the isotope to active brain regions. Alternatively, it is also possible to attach a radioactive label to molecules that bind to specific neurotransmitter receptors, which allows the researcher to actively monitor their distribution in the brain.
Another popular indirect measure of brain activity is functional Magnetic Resonance Imaging (fMRI). It uses strong magnetic field which evoke responses in the magnetic signatures of oxygenated and deoxygenated hemoglobin in the blood flow, which allows for them to be monitored.
Indirect measures like these come with a stark methodological shortcoming. By their nature, they can only observe changes in distribution of oxygen/glucose or neurotransmitters, meaning that they cannot pick up on brain activity that does not involve changes in blood flow. By extension, any not sufficiently large-scale changes in brain activity would not even be registered by indirect measures. Beyond this, due to the seconds-long time delay between neural activity and rerouting of blood flow, indirect measures cannot fully capture the temporal architecture of the brain, which would require millisecond precision.
Direct Measures
For most of my research, I have relied on direct measures of brain activity. Most commonly, I use electroencephalography (EEG), which reads the electrical activity of groups of neurons from the scalp. As it is picked up with sensors placed on the head of the participant, the signal is very weak and only large-scale changes in activity are detectable, as the cumulative signal of neural firings would be too attenuated before reaching the scalp otherwise.
Because of this, and because of the dispersed spread of electrical activity generated by groups of neurons, EEG readings are very spatially imprecise and so do not allow for accurate localisation of the origin of the change in brain activity aside from very rough scalp topologies. The main advantage of EEG lies in the high temporal resolution, giving an account of the brains' temporal architecture with millisecond precision instead of the seconds-long lag of indirect measures.
Another approach called magnetoencephalography (MEG) offers the, to this date, perhaps best non-invasive compromise between the high spatial resolution of fMRI and PET and the high temporal resolution of EEG by tracking the brains' magnetic activity. Since every electric field always comes with a magnetic one, it is possible to read the magnetic activity generated by electrical discharges of neurons, rather than the electric activity itself. Reading magnetic activity holds the advantage that a magnetic signal can travel through the brain and scalp with a low degree of distortion, while the origin of the electrical signal is distorted by current conductivity caused by cerebrospinal fluid or skin. Because of this, MEG retains the temporal resolution of EEG, but offers far higher spatial resolution.
Invasive Measures
To further enhance signal quality and precision in locating the source of the picked-up signal, it is also possible to implant neural activity-sensing microelectrode arrays (MEAs) into the brain via neurosurgery. There are three major types of MEAs which are currently in use: Microwire MEAs that use thin wires made out of steel or tungsten to triangulate the position of neurons they are monitoring, Michigan arrays that provide a higher density recording of brain activity by means of arrays of neuron-sized shanks with flat electrodes on their sides, and Utah arrays which feature an array of needle-like shanks with electrodes on their tips.
The process of implanting MEAs is similar to the neurosurgery procedure I described in my article on Brain Stimulation and requires utmost precision. If the MEA is placed wrongly, or the surface area of the MEA is so big it squashes the soft jelly-like structure of the brain, this can cause damage, which, at best diminishes the MEA signal quality, and at worst causes serious injury to the patient.
This is especially true if the MEA is made out of metal, which is a popular choice due to its conductive qualities. Regardless, even if implanted correctly, MEAs will, at some point, bring about adverse reactions in patients, as it continues to irritate the brain surrounding it, causing inflammation which then negatively affects the MEA function. At this point, or when the battery or other components powering the array need to be replaced, additional surgical procedures are needed – usually every few years.
An alternative to MEAs are electrocorticography (ECOG) electrode arrays. They consist of a somewhat flexible sheet (usually silicone-based) with embedded flat disc-shaped electrodes which do not penetrate neural tissue but simply sit on top of the brain surface. In consequence, they do not provoke the same immune reaction that MEAs are prone to evoke over time. However, given that a single cubic millimetre of the human cerebral cortex already contains about 50k neurons with 6k synapses with neighbouring cells (making for 300 million interconnections), even small ECOG electrodes of about 2.3 mm have far worse spatial resolution than MEAs, which are able to monitor individual neurons.
The shortcomings in ECOG arrays point to the need for innovation in MEAs – both in terms of the arrays used and in their implantation in order to reduce associated risks. In the future we might be using conductive hydrogels instead of metal electrodes, which could wrap around neural tissue instead of penetrating it, hence reducing the risk of the brain rejecting the implant.
For now, however, some of the most interesting advances culminated in research by Neuralink. Building on work done by DARPA, the company has developed thin flexible polymer-based "Neural Lace" electrode arrays which are powered wirelessly by a battery that sits outside the head in a small device behind the ear, reducing the need for surgical procedures when the battery needs to be replaced.
Implanting the Neural Lace arrays is also much simpler: First, the arrays are threaded onto small needles. Then, a surgical robot uses computer vision to find their target region and shoots the needles into the brain. This automatic insertion of one "Neural Lace" thread takes less than an hour – much faster than traditional neurosurgery. However, the speed at which the needles are shot into the brain is also worrisome, as rapid thread insertion is likely to damage surrounding brain tissue. This would ultimately negatively affect the signal quality picked up by the electrode array. In fact, data released by Neuralink indicates that this is the case, meaning that some more work needs to be done before the technology is ready for use with human participants. Still, the future looks promising!
Practical Applications
These means of observing the brain in action aid our understanding of its architecture and mechanisms. Practical applications outside a research-context are numerous. For example, EEG analysis can serve as a diagnostic, identifying risk for neurodegenerative diseases years in advance (Schreiter-Gasser, Gasser & Ziegler, 1993), help to identify anxiety and depressive disorders in patients (Kawe, Shadli & McNaughton, 2019), or inform personalized medicine (Arns & Oblbrich, 2014).
Aside from being a diagnostic tool, brain imaging can also serve as means of therapy, as I detailed in my article on Biofeedback. Classical forms of therapy often deal with the attempt of bringing a previously unconscious mental process that troubles the patient into their awareness, so it can be consciously controlled. Clinicians can use EEG and other brain imaging modalities for the same end, by taking a real-time measure of a pathological brain activity pattern of the patient, presenting it to them, and giving guidance on what a healthy brain activity pattern looks like in comparison.
With this knowledge, the patient can then observe the real-time measure and, thanks to this immediate feedback, attempt to normalize their brain activity. This approach has been shown to yield long-term benefits ADHD, for example, where a two-year follow-up study found not just sustained normalized brain activity, but also behavioral improvements (Becerra et al., 2017).
Finally, monitoring brain activity in real time also allows researchers to build so-called brain-computer interfaces (BCIs) which allow for the control of dedicated software or hardware with nothing but thought. For example, we've had some success in reconstructing imagined speech (Deng & D'Zmura, 2013; Kumar et al., 2018) or imagined imagery (Seoane, Gabler & Blankertz, 2014) and use it as software input.
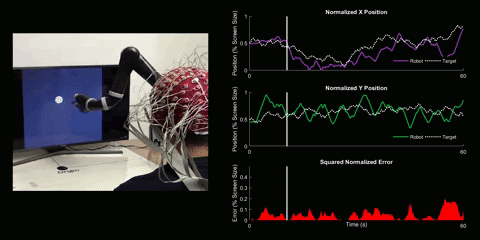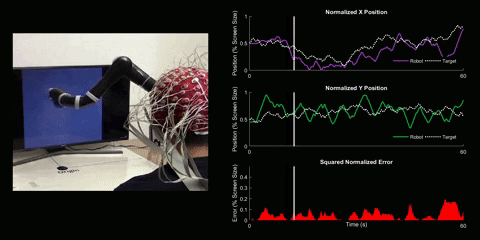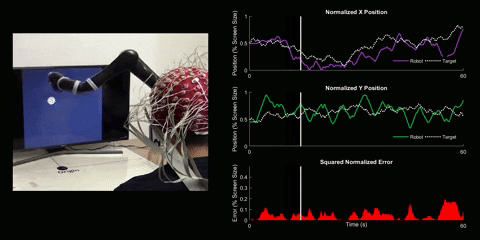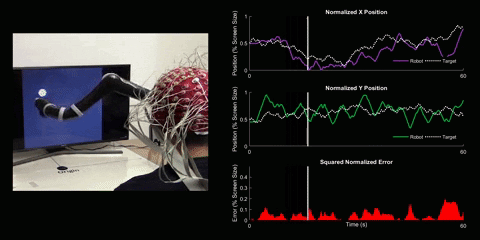
Brain-Computer Interface Control
Using 128 EEG electrodes placed on the scalp of the participant, brain activity that accompanies imagined movements is monitored. The real-time signal is then turned into input commands for a robotic arm and participants are asked to use their thoughts to make the robotic arm follow a white dot on screen.
While this is obviously exciting because it might allow for new means of communicating both with software and – possibly – each other, this technology will perhaps do the greatest goods in a context where people are unable to interact with the world in ways that we might take for granted. Aside from enhancing the quality of prosthetics by allowing for them to be moved in manifold ways by brain signals (Buch et al., 2018), applying BCIs in cases of disorders of consciousness seems like a worthwhile cause to me.
Not just can BCIs be used to assess the degree of consciousness (Colombo et al., 2019), but also allow patients with locked-in syndrome for a means to communicate with the outside world when they are unable to move any part, or most, of their body (Annen, Laureys & Gosseries, 2020).
In sum, brain imaging can be used to help us learn more about the brain, diagnose and fix its problems and serve as a new means to communicate with dedicated software. Perhaps, in the future, brain imaging might also allow for us to communicate in novel ways with each other: an electrode array might read brain activity in one person and send the signals to an electrode array in the brain of another, which then proceeds to stimulate their brain accordingly, enabling brain-to-brain communication – a concept that has already been shown to work in some early research (Grau et al., 2014). Regardless of speculations on future applications, there are ample ways of applying brain imaging technology to learn more about ourselves, each other and to do good. So let's get to it!
Sources
Sources
Annen, J., Laureys, S. and Gosseries, O., 2020. Brain-computer interfaces for consciousness assessment and communication in severely brain-injured patients. In Handbook of Clinical Neurology (Vol. 168, pp. 137-152). Elsevier.
Arns, M. and Olbrich, S., 2014. Personalized medicine in ADHD and depression: use of pharmaco-EEG. In Electrophysiology and Psychophysiology in Psychiatry and Psychopharmacology (pp. 345-370). Springer, Cham.
Becerra, J., Fernandez, T., Harmony, T., Caballero, M.I., Garcia, F., Fernandez-Bouzas, A., Santiago-Rodríguez, E. and Prado-Alcalá, R.A., 2006. Follow-up study of learning-disabled children treated with neurofeedback or placebo. Clinical EEG and neuroscience, 37(3), pp.198-203.
Buch, V.P., Richardson, A.G., Brandon, C., Stiso, J., Khattak, M.N., Bassett, D.S. and Lucas, T.H., 2018. Network brain-computer interface (nBCI): An alternative approach for cognitive prosthetics. Frontiers in neuroscience, 12, p.790.
Colombo, M.A., Napolitani, M., Boly, M., Gosseries, O., Casarotto, S., Rosanova, M., Brichant, J.F., Boveroux, P., Rex, S., Laureys, S. and Massimini, M., 2019. The spectral exponent of the resting EEG indexes the presence of consciousness during unresponsiveness induced by propofol, xenon, and ketamine. NeuroImage, 189, pp.631-644.
Deng, S., Srinivasan, R. and D'Zmura, M., 2013. Cortical signatures of heard and imagined speech envelopes. CALIFORNIA UNIV IRVINE DEPT OF COGNITIVE SCIENCES.
Grau, C., Ginhoux, R., Riera, A., Nguyen, T.L., Chauvat, H., Berg, M., Amengual, J.L., Pascual-Leone, A. and Ruffini, G., 2014. Conscious brain-to-brain communication in humans using non-invasive technologies. PloS one, 9(8).
Kawe, T.N., Shadli, S.M. and McNaughton, N., 2019. Higuchi's fractal dimension, but not frontal or posterior alpha asymmetry, predicts PID-5 anxiousness more than depressivity. Scientific Reports, 9(1), pp.1-7.
Kumar, P., Saini, R., Roy, P.P., Sahu, P.K. and Dogra, D.P., 2018. Envisioned speech recognition using EEG sensors. Personal and Ubiquitous Computing, 22(1), pp.185-199.
Schreiter-Gasser, U., Gasser, T. and Ziegler, P., 1993. Quantitative EEG analysis in early onset Alzheimer's disease: a controlled study. Electroencephalography and clinical neurophysiology, 86(1), pp.15-22.
Seoane, L.F., Gabler, S. and Blankertz, B., 2014. Images from the Mind: BCI image evolution based on RSVP of polygon primitives. CoRR.
